2024 Tus sau: Howard Calhoun | [email protected]. Kawg hloov kho: 2023-12-17 10:28
Kev sib cais hluav taws xob ua lub luag haujlwm loj hauv peb lub neej, txawm tias peb feem ntau tsis xav txog nws. Nws yog nrog qhov tshwm sim no uas cov hluav taws xob conductivity ntawm ntsev, acids thiab cov hauv paus hauv cov kua nruab nrab yog txuam nrog. Los ntawm thawj lub plawv dhia los ntawm "nyob" hluav taws xob nyob rau hauv tib neeg lub cev, uas yog yim caum feem pua kua kua, rau lub tsheb, xov tooj ntawm tes thiab cov players, cov roj teeb uas tseem ceeb yog cov roj teeb hluav taws xob, hluav taws xob dissociation yog invisibly muaj nyob txhua qhov chaw nyob ze peb.
Nyob rau hauv cov vats loj heev uas tawm cov pa phem los ntawm bauxite melted ntawm qhov kub thiab txias, "tis" hlau - txhuas yog tau los ntawm electrolysis. Txhua yam nyob ib puag ncig peb, los ntawm chrome radiator grilles rau nyiaj-plated tsej hauv peb pob ntseg, ib zauglos yog ntsib nrog kev daws teeb meem los yog molten ntsev, thiab li no nrog qhov tshwm sim. Nws tsis yog rau tsis muaj dab tsi uas hluav taws xob dissociation yog kawm los ntawm tag nrho cov ceg ntawm science - electrochemistry.
Thaum yaj, cov molecules ntawm cov kua ua kua ua kua nkag mus rau hauv cov tshuaj lom neeg nrog cov molecules ntawm cov tshuaj yaj, tsim cov solvates. Nyob rau hauv ib tug aqueous tov, ntsev, acids thiab bases yog feem ntau raug rau dissociation. Raws li cov txheej txheem no, cov kua dej molecules tuaj yeem decompose rau hauv ions. Piv txwv li, nyob rau hauv kev cuam tshuam ntawm cov kuab tshuaj aqueous, Na+ thiab CI- ions hauv NaCl ionic siv lead ua dhau mus rau qhov hnyav nruab nrab hauv ib qho. tshiab zoo ntawm solvated (hydrated) particles.
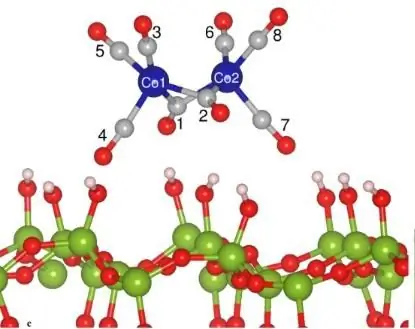
Qhov tshwm sim no, uas yog qhov tseem ceeb ntawm cov txheej txheem ua tiav lossis ib nrab decomposition ntawm cov tshuaj yaj mus rau hauv ions raws li qhov tshwm sim ntawm qhov hnyav, hu ua "hluav taws xob dissociation". Cov txheej txheem no tseem ceeb heev rau electrochemistry. Qhov tseem ceeb tshaj plaws yog qhov tseeb tias dissociation ntawm complex multicomponent systems yog characterized by stepwise flow. Nrog rau qhov tshwm sim no, kuj tseem muaj qhov nce ntxiv ntawm cov ions hauv cov tshuaj, uas txawv cov tshuaj electrolytic los ntawm cov uas tsis yog electrolytic.
Nyob rau hauv cov txheej txheem ntawm electrolysis, ions muaj cov kev taw qhia meej ntawm kev txav: cov khoom uas muaj tus nqi zoo (cations) - mus rau qhov tsis zoo electrode, hu ua cathode, thiab zoo ions (anions) - rau lub anode, electrode nrog tus nqi rov qab, qhov twg lawv tawm. Cations raug txo thiab anions yog oxidized. Yog li ntawd, dissociation yog ib tug reversible txheej txheem.
Ib qho ntawm cov yam ntxwv tseem ceeb ntawm cov txheej txheem electrochemical no yog qib ntawm electrolytic dissociation, uas yog qhia raws li qhov piv ntawm cov hydrated hais rau tag nrho cov molecules ntawm cov khoom yaj. Qhov ntsuas no siab dua, qhov muaj zog ntawm electrolyte yog cov khoom no. Raws li lub hauv paus no, txhua yam khoom raug muab faib ua qaug zog, nruab nrab lub zog thiab muaj zog electrolytes.
Lub degree ntawm dissociation nyob ntawm cov nram qab no yam: a) qhov ntawm lub solute; b) qhov xwm ntawm cov kuab tshuaj, nws cov dielectric tas li thiab polarity; c) concentration ntawm kev daws (qhov ntsuas no qis dua, qhov ntau dua qhov kev sib cais); d) qhov kub ntawm qhov nruab nrab dissolving. Piv txwv li, kev sib cais ntawm acetic acid tuaj yeem qhia los ntawm cov qauv hauv qab no:
CH3COOH+ +3COO-
Muaj zog electrolytes dissociate yuav luag irreversibly, txij li thaum lawv cov aqueous tov tsis muaj cov thawj molecules thiab tsis-hydrated ions. Nws tseem yuav tsum tau ntxiv tias txhua yam khoom uas muaj ionic thiab covalent polar hom tshuaj tiv thaiv yog raug rau cov txheej txheem dissociation. Txoj kev xav ntawm electrolytic dissociation yog tsim los ntawm Swedish physicist thiab chemist Svante Arrhenius nyob rau hauv 1887.
Pom zoo:
Yuav them nqi hluav taws xob li cas hauv Is Taws Nem? Kev them nyiaj hluav taws xob ntawm tus kheej tus account hauv Internet

Tom qab Is Taws Nem tau ruaj khov thiab sib koom ua ke rau hauv kev muaj tiag Lavxias, kev lag luam nyiaj txiag hauv online tau tso tseg tsis yog ib qho khoom tshwj xeeb rau tib neeg. Kev ua haujlwm them nyiaj online, txawm tias rau tus neeg siv PC tsis muaj kev paub, yog qhov yooj yim heev. Hauv kab lus no koj tuaj yeem pom cov lus qhia ntxaws txog yuav ua li cas koj tuaj yeem them hluav taws xob siv Is Taws Nem
Kev hloov pauv ntawm thermal zog rau hauv hluav taws xob hluav taws xob nrog kev ua haujlwm siab: txoj hauv kev thiab khoom siv

Muaj kev txhawj xeeb thoob plaws ntiaj teb txog qhov kev puas tsuaj poob qis hauv qib ntawm cov khoom siv hluav taws xob uas xav tau rau lub neej niaj hnub, xws li roj, roj thiab roj. Txawm li cas los xij, qhov tseeb no ua rau muaj kev txhim kho cov thev naus laus zis tshiab raws li kev siv lwm yam khoom siv ntuj tsim: hnub ci zog, hydropower, cua zog, bioenergy, geothermal zog. Qhov no yog nrov nyob rau hauv tsab xov xwm
Kev lag luam hluav taws xob ntawm Russia. Kev loj hlob ntawm kev lag luam hluav taws xob
Kev lag luam hluav taws xob hauv tsev tau dhau nws 50 xyoos. Nws pib hauv USSR, thaum tsim cov chaw tshawb fawb thiab cov tuam txhab high-tech. Muaj ups thiab downs raws txoj kev, thiab oblivion
Kev them nqi hluav taws xob hauv Is Taws Nem. Yuav them cov nqi hluav taws xob li cas hauv online

Tsis tas yuav hais ntau qhov tseeb tias mus rau lub txhab nyiaj them nqi hluav taws xob yog qhov dhuav heev. Thiab muaj cov laj thawj zoo heev rau qhov no. Tab sis koj tuaj yeem them nqi hluav taws xob hauv Internet. Hauv qhov kev tshuaj xyuas no, qhov no yog qhov tseeb uas yuav tau tham txog
Yuav siv cov tshuaj tua hluav taws dab tsi los tua hluav taws xob thaum muaj hluav taws kub?

Hluav taws xob thaiv hluav taws xob yog qhov txaus ntshai heev. Yog li ntawd, cov tshuaj tua hluav taws yuav tsum muaj txiaj ntsig txaus los tua nws. Cov ntsiab lus no yog qhov tseem ceeb rau txhua lub koom haum lossis kev lag luam uas muaj cov khoom siv hluav taws xob. Cov cai tswj kev nyab xeeb hluav taws xav tau cov tshuaj tua hluav taws. Lawv yuav tsum yog dab tsi? Xav txog yam kev tua hluav taws uas koj tuaj yeem tua hluav taws xob, suav nrog lawv cov yam ntxwv thiab lub xeev tam sim no ntawm daim phiaj hluav taws xob








